A 2-Billion-Year-Old Nuclear Reactor
The incredible yet little-known natural fission reactors that can teach us about waste management
In 1972, French scientists at the Pierrelatte uranium enrichment facility noticed an anomaly in the samples from a mine near the region of Oklo in Gabon, located just below the equator in West Africa. The samples had a lower-than-expected amount of uranium-235 (U-235), the element used in fission power plants and in nuclear bombs. They contained only 0.717% of the fissionable isotope, instead of the expected 0.72%.
That might seem like a very small discrepancy, but the distribution of uranium isotopes is remarkably constant throughout earth’s crust. No matter where it is mined, uranium ore consistently maintains the same ratio of uranium-235 to uranium-238.
When scientists went back to test old samples, they found the lower-than-expected ratio to be present in uranium ore samples dating back to 1970, leading to the conclusion that up to 200 kilograms of U-235 from Gabon were unaccounted for. Theories ranged from sample contamination — unlikely, given that all samples showed the same unusual isotopic ratio — to a more alarming possibility of sophisticated uranium theft during the height of the nuclear arms race.
Eventually, enough evidence was gathered to determine that the uranium in the samples had already undergone fission in a natural setting. In other words, what scientists had been looking at were remnants of nuclear waste produced in a natural fission reactor almost two billion years ago.
How could that be?
How a chain fission reaction happens
Fission typically occurs when a U-235 nucleus absorbs a neutron, briefly transforming into uranium-236 (U-236), an unstable isotope that swiftly splits into two lighter atoms. These fission products are not always the same elements, but typically include barium (Ba) and krypton (Kr). The process also releases 2 or 3 neutrons, gamma radiation, and a significant amount of energy. This energy is what drives the heat in nuclear reactors or the explosion in nuclear weapons.
The newly released neutrons, in turn, sustain the chain reaction. If at least one of them is absorbed by another U-235 nucleus, that atom in turn splits, releases more energy and neutrons, and the process continues. If, on average, more than one neutron per fission leads to additional fission events, the reaction will become self-sustaining and can even accelerate. A controlled chain reaction is the most efficient (as well as the cleanest, and the safest) mechanism that exists today for generating electricity. On the flipside, if a significant amount of fissionable material is present, an uncontrolled chain reaction is more commonly known as a nuclear bomb detonation.

Sustaining and controlling such a process in a reactor core requires that a few conditions be present:
A high concentration of fissionable material. Basically, there must be enough of a critical mass of atoms that can absorb neutrons and then split to produce more neutrons, and so on, to keep the reaction going. The uranium fuel in most nuclear reactors has a U-235 concentration of 3% to 5%, whereas nuclear weapons contain over 90% of the stuff1.
A moderator. The neutrons released during fission are very fast, with high kinetic energy. Fast neutrons are less likely to be absorbed and tend to escape, leading the chain reaction to fizzle out. A moderator such as water or graphite slows down these fast neutrons to lower energies (thus becoming thermal neutrons), increasing the probability that they will be absorbed by other nuclei.
An inhibitor. Elements like boron or cadmium can absorb neutrons without undergoing fission, thus reducing their availability in the core. Reactors use control rods made of these materials that can be inserted into the core to slow down or even stop a chain reaction, essentially by sucking up all the free neutrons.
How it happened at Oklo
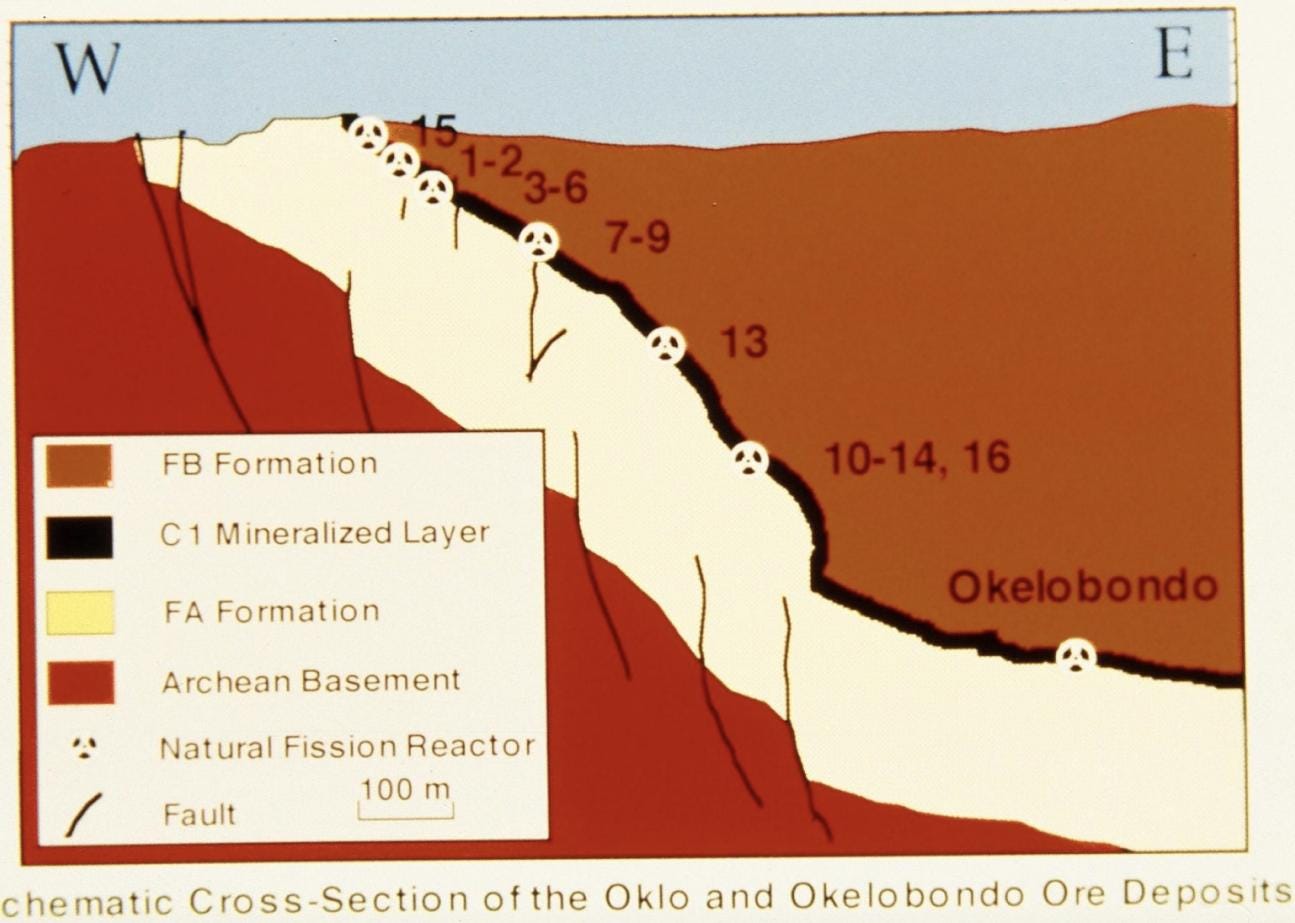
An analysis of the samples from Gabon revealed 17 natural nuclear reactor sites that functioned intermittently for about 150,000 years, with an average power output of about 100 kilowatts — paltry, compared to modern commercial power plants that can exceed 1,000,000 kilowatts. How did the Oklo reactors encounter the conditions that enabled them to operate for such a long time?
Two billion years ago, the proportion of U-235 in uranium ore was about 3.6%, a concentration similar to the enriched material that powers our fission power plants, and much higher than in the ore mined today. The composition of uranium ore keeps changing because U-235 decays at a much faster rate than the more common isotope U-238 (when the Earth was formed, that proportion was higher than 30%.)
With that, it’s pretty clear that two billion years ago it would have been possible for enough fissionable material to accumulate somewhere on the planet2, and indeed it did, as evidenced by the many uranium reserves spread across the world. And yet, evidence for fission activity was only ever found in Oklo and nowhere else. The reason appears to be that the Oklo deposits had water that was free of impurities and in just the right amount to act as a moderator:
The medium was probably saturated with water which would have further overmoderated the neutrons. If the ore became chain-reacting in this condition, it would boil off the water until it became slightly undermoderated, eventually moving back to optimum moderation.
In addition, there’s no evidence of elements that could have acted as inhibitors, thus enabling the fission process to continue at a slow burn for several millennia.
What about the waste?
I’ve researched enough about nuclear waste to understand the angst it causes in many people. Fresh out of the reactor core, as it would have been in Oklo 2 billion years ago, spent fuel is indeed extremely hazardous. It must be handled with immense care because it can inflict a lethal dose of radiation in seconds — fortunately, it is likely the most securely managed substance on the planet, with no significant accidents ever recorded. And the stuff stays hazardous for a long time. It can take more than a hundred thousand years for fission products in the spent fuel to return to the background levels of radiation found in nature3.
Because the natural reactors operated for many millennia, they produced radioactive fission products that remained uncontained and exposed to the elements for hundreds of thousands of years. So, what happened to this waste?
After billions of years of geologic activity, the radioactive material shifted by only a few centimeters, showing no evidence of having contaminated the environment around it. This natural experiment has helped scientists validate models of nuclear waste behavior over long timescales. These models are critical for designing the dry casks that store spent fuel on the surface, as well as the underground tunnels in Deep Geological Repositories (DGRs). The world’s first DGR, set to start operating in Finland before the end of this year, uses multiple geologic and engineered barriers to isolate highly radioactive material. It will probably do so for eternity — not unlike Oklo.
Resources
This research paper from just a few years after the Oklo discovery goes a lot deeper. There’s a TikTok version too, of course.
Uranium enrichment is what gets the concentration from the 0.72% in mined ore to the higher levels needed by fission reactors and, if you’re into that kind of thing, weapons capable of levelling a large city in seconds.
The astute reader might ask why there weren’t natural nuclear reactors popping up all over the planet even longer ago, when Earth had a lot more U-235. The answer there has to do with the conditions not being right for enough of it to accumulate in one place. As most other elements in Earth’s early history, uranium was scattered across the crust in trace amounts, trapped within rocks. To end up with high concentrations, you need oxygen in the mix to make uranium soluble in water, at which point it eventually gets carried around and deposited in one place. And oxygen wasn’t abundant on our planet until the Great Oxidation Event, around 2.4-2.1 billion years ago.
It’s important to note that radioactive decay is an exponential process. It happens very rapidly at the start, and it has a very long tail. Therefore, even though it can take up to 200,000 years for radiation in spent fuel to return to levels found in nature, after about 600 years most of the dangerous radioactivity in the material has dissipated, at which point it cannot do much harm at a distance. I’ve written about it at length here.





Fascinating. Thank you!